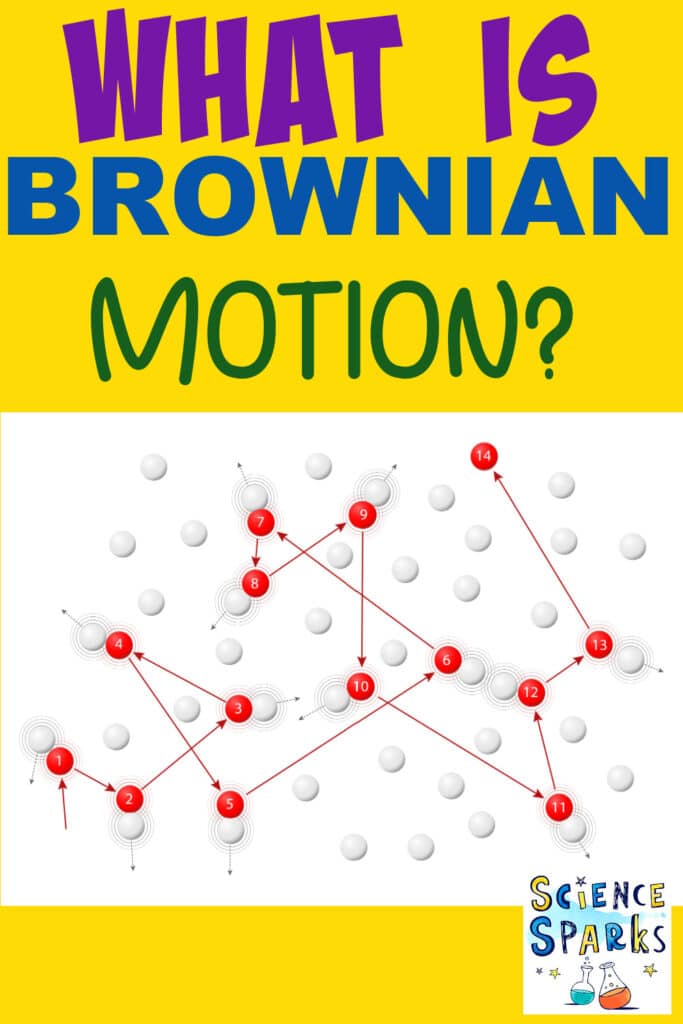
Brownian Motion is the random movement of particles suspended in a liquid or gas. The movement is caused by particles colliding with each other.
Brownian Motion is named after a botanist, Robert Brown, who noticed pollen grains moving around randomly in water under a microscope. Even though pollen grains are much larger than water molecules, there were still enough collisions for them to be moved.
Robert Brown didn’t understand why the pollen grains he observed were moving, but he was the first scientist to document this kind of movement. It was Albert Einstein who explained that water molecules were moving the pollen grains, and Brownian Motion was used as evidence for the kinetic theory of matter.
This video explains Brownian Motion brilliantly!
What’s the difference between Brownian Motion and Diffusion?
- Diffusion is the movement of particles from a high to a low concentration. The particles have a direction of travel. In Brownian Motion, the movement of particles is random.
- Diffusion occurs when there is a concentration gradient. Brownian Motion occurs due to the movement of other particles in the liquid or gas.
Science concepts
Diffusion
Brownian Motion
Last Updated on June 26, 2023 by Emma Vanstone
